Dear Devotees,
The following article has been written and researched by our inhouse team of medical experts. The article is a concise overview of what vaccines are, the history of vaccination, specific information about the Pfizer mRNA Covid-19 vaccine and also addresses some of the concerns and misunderstandings there may be around it.
We trust that you will find this article informative and we will also attempt to address any further questions that may arise. The article is hyperlinked for ease of navigation and review so as to enable you to select sections which may be of more interest to you.
Table of Contents
- History and evolution of vaccines
- COVID-19 and its vaccine
- About COVID-19
- Understanding the principle underlying COVID-19 mRNA vaccine
- FDA approved COVID-19 vaccine
- Safety concerns regarding COVID-19 vaccine
- Concern 1: COVID-19 vaccine has toxic ingredients.
- Concern 2: COVID-19 vaccine has recombinant genetic material that can modify or change my DNA causing an eternal genetic disorder.
- Concern 3: The Pfizer-BioNTech COVID-19 vaccine is not effective.
- Concern 4: COVID-19 vaccine had caused severe life-threatening complications including face paralysis and death during the trials, but the vaccine was still approved and released in the market.
- Concern 5: COVID-19 vaccine will give me COVID-19.
- Concern 6: FDA approved Pfizer-BioNTech COVID-19 vaccine has HIV.
- Concern 7: The COVID-19 vaccine cannot give long-term protection because the COVID-19 virus is rapidly mutating/changing. New strain has emerged in the UK, which is more contagious, and the present day approved vaccine will not be able to protect from the infection with this new strain.
- Concern 8: COVID-19 vaccine is not safe, it can cause injury, reversals, and adverse reactions.
- Bibliography
What is a vaccine and how does it work?
The body’s immune system functions to fight the disease and protect us by:
a) Recognizing the foreign substances in the body (like bacteria or their toxins, virus, parasites, fungi etc, commonly called as germs or pathogens)
b) Developing a defense against them.
Mounting of this defense against foreign invaders is called as an immune response. This immune response is nothing but generation of antibodies, which are molecules that destroy the germs. Immunity is the ability of the human body to tolerate the material native to the body (“self”), and to eliminate foreign (“non-self”) material.
Vaccines work on the same principle. A vaccine contains the same germs that cause the disease; however, the injected germs are either inactivated or exposure to them is controlled. Therefore, vaccinations cheat the immune system to generate antibodies against the germs so that when the vaccinated individual is later infected with (or exposed to) the actual germs from the environment, the pre-existing antibodies can destroy those germs before they can cause disease.
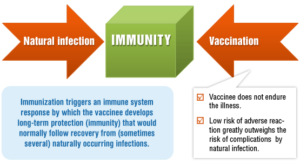
Thus, there are two ways of acquiring protection from the germs — by natural infection or by vaccination. Natural infections and vaccines produce a very similar end result — immunity.
History and evolution of vaccines
Humans, animals, and food are moving around the world more frequently and more easily than ever before; thus, carrying disease causing agents with them. In today’s globalized era of unparalleled human movement and interaction, diseases can spread quickly and are no longer contained to isolated geographical areas. What used to be an epidemic (a disease that affects a large number of people within a community, population, or region) is becoming pandemic (a global epidemic that is spread over multiple countries or continents). Initially, new infectious diseases could spread only as fast and far as people could walk. Then as fast and far as horses could gallop and ships could sail. With the advent of aviation, the last five centuries have seen more new diseases than ever before become potential pandemic.

 Thus, with a clear impact of globalization on dissemination of communicable diseases, it is of paramount importance to develop vaccines as the “Weapons of mass protection”. And it is quite evident from the table that the concept of vaccines is not new to humanity. Vaccination has been an integral part of our system for hundreds of years.
Thus, with a clear impact of globalization on dissemination of communicable diseases, it is of paramount importance to develop vaccines as the “Weapons of mass protection”. And it is quite evident from the table that the concept of vaccines is not new to humanity. Vaccination has been an integral part of our system for hundreds of years.
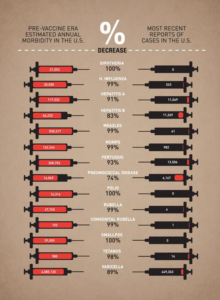
Interestingly, the CDC (Center for Disease Control and prevention) compares the morbidities (how many people became sick) of what were once very common infectious diseases in the USA, from the pre- and post-vaccination era. The data clearly reveals the versatility of vaccination. There is no smallpox, no polio, no diphtheria, almost no measles, dramatically less chickenpox (also known as varicella) and H. influenza (that’s not flu, but a bacterium that causes deadly meningitis).
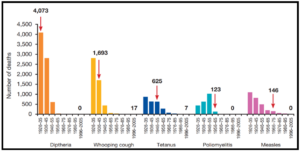
Not only that, but vaccination has also been able to lower the mortality (the death rate) of numerous diseases significantly. The red arrow in the graph indicates when a vaccine was introduced (Image adapted from: Australian Academy of Science).
When enough people in a given population are vaccinated, the spread of disease from one person to another is stopped or at least contained. This is called ‘herd immunity’ or “community immunity”. It helps to indirectly protect even those individuals who are unable to receive vaccination (newborns or immunocompromised or due to any other reason).
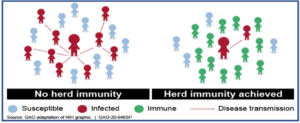
COVID-19 and its vaccine
About COVID-19
Coronavirus disease 2019 (COVID-19) is caused by a new coronavirus first identified in Wuhan, China, in December 2019. Coronaviruses are named for the crown-like spikes on their surfaces. In COVID-19, ‘CO’ stands for ‘corona,’ ‘VI’ for ‘virus,’ and ‘D’ for disease. Because it is a new virus, scientists are learning more each day. Although most people who have COVID-19 have mild symptoms, COVID-19 can also cause severe illness and even death. Some groups, including older adults and people who have certain underlying medical conditions, are at increased risk of severe illness.
The SARS-CoV-2 pandemic presents an extraordinary challenge to global health and, as of November 30, 2020, it has caused more than 60 million infections and claimed the lives of 1.5 million people worldwide. In the United States, over 13 million cases have been reported to the Centers for Disease Control and Prevention (CDC), with over 260,000 deaths. Confirmed cases and mortality continue to rise globally. Unlike the flu virus, as the figure depicts, COVID-19 is more contagious (R0>2), more deadly and thus has a greater potential to overwhelm our health care system. COVID-19 virus has a longer incubation time (1-14 days as opposed to 1-4 days for the flu virus). Incubation time is the time between the exposure and the manifestation of symptoms. Thus, a person will take longer to realize that he/she has contracted the virus and will keep spreading it.
Understanding the principle underlying COVID-19 mRNA vaccine
mRNA vaccines represent a new generation technology. The conventional traditional vaccines put a weakened or inactivated germ into our bodies to trick the immune system to build immunity. The mRNA vaccines instead have strands of genetic material called mRNA inside a special coating. That coating protects the mRNA from enzymes in the body that would otherwise break it down. It also helps the mRNA enter the muscle cells near the vaccination site.
The mRNA provides a coded information to our cells how to make a protein that triggers an immune response inside our bodies. In the case of COVID-19, the mRNA vaccine gives instructions to our cells to make a harmless protein called as the “spike protein.” The spike protein is found on the surface of the virus that causes COVID-19. Once the instructions (mRNA) are inside the muscle cells, the cells use them to make the spike protein. After the protein is made, the cell breaks down the instructions (mRNA) and gets rid of them. Our immune system recognizes that the spike protein is foreign (does not belong there) and begins making antibodies against the viral protein. Thus, our bodies become equipped with the antibodies against the spike protein of the COVID-19 virus so that we are protected from the COVID-19 infection in future.
Although mRNA technology is new, it is not unknown. As of now, there are currently no licensed mRNA vaccines in the United States except COVID-19. mRNA vaccines have several benefits compared to other types of vaccines including use of a non-infectious element, shorter manufacturing times, ease of production and scale up.
FDA approved COVID-19 vaccine
Pfizer, in partnership with BioNTech Manufacturing GmbH, has developed a vaccine to prevent COVID-19 which is based on the SARS-CoV-2 spike glycoprotein (S) antigen encoded by RNA and formulated in lipid nanoparticles (LNP). The Pfizer-BioNTech COVID-19 vaccine is administered intramuscularly as a 2-dose series spaced 21 days apart. The vaccine is supplied as a multi-dose vial (5 doses) containing a frozen suspension (-80°C to -60°C or – 112°F to -76°F) that must be thawed and diluted with 1.8 mL of sterile 0.9% sodium chloride (salt), allowing for five 0.3 mL doses. After dilution, the multiple-dose vials must be stored between 2°C to 25°C (35°F to 77°F) and used within 6 hours from the time of dilution.
Safety concerns regarding COVID-19 vaccine
Concern 1: COVID-19 vaccine has toxic ingredients.
As per the fact sheet released by the FDA, none of the ingredients have been shown or proven to be toxic to the humans. The vaccine is also preservative free. Specifically, the Pfizer BioNTech COVID-19 vaccine includes the following ingredients:
- mRNA
that encodes for the spike protein of the COVID-19 virus
- Lipids/fats
((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3- phosphocholine, and cholesterol
- Salts
Potassium chloride, Monobasic potassium phosphate, Sodium chloride, Dibasic sodium phosphate dihydrate
- Sugar
Sucrose
Concern 2: COVID-19 vaccine has recombinant genetic material that can modify or change my DNA causing an eternal genetic disorder.
There is an ongoing theory and uninformed claims that COVID-19 vaccine will somehow change our DNA, causing fear in the common masses. This has become a popular rumor aired regularly on social media and is being circulated via other online portals. It is understandable that the coronavirus vaccine represents some of the newest vaccine technology and thus can bring skepticism and erroneous claims; however, as per the basic fundamentals of science, the figure below explains how COVID-19 vaccine based on mRNA cannot access our DNA under any circumstances.
- Each cell in our body has a nucleus and a cytoplasm.
- DNA is the most important molecule that carries the blueprint or code or instructions to produce proteins in our body. This DNA carrying the codes resides inside the nucleus so that it is protected.
- However, DNA by itself cannot make proteins inside the nucleus. This is because the machinery for production of proteins is not present in the nucleus but is present in the cytoplasm.
- So how does the information from DNA (in the nucleus) is decoded into proteins?
- This is accomplished by an intermediate molecule called messenger RNA or mRNA. As the name suggests, mRNA acts as a messenger that first copies the information from DNA in the nucleus (a process called “transcription”) and then travels to the cytoplasm carrying the information for production of proteins in the cytoplasm.
- The mRNA is then decoded to produce proteins using machinery that is present in the cytoplasm (a process called “translation”).
- The COVID-19 mRNA vaccine delivers the mRNA containing the code for making COVID-19 spike protein, straight into the cytoplasm.
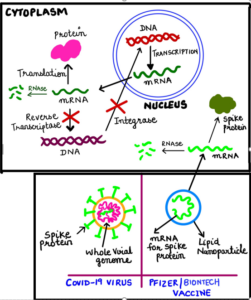 Like for any other protein, this mRNA will also make COVID-19 spike protein using the cytoplasmic machinery. However, this protein will be recognized as foreign by our immune system, leading to the generation of antibodies. Next time, when we encounter actual COVID-19 virus from the environment, these antibodies will destroy the disease-causing virus offering us protection or immunity.
Like for any other protein, this mRNA will also make COVID-19 spike protein using the cytoplasmic machinery. However, this protein will be recognized as foreign by our immune system, leading to the generation of antibodies. Next time, when we encounter actual COVID-19 virus from the environment, these antibodies will destroy the disease-causing virus offering us protection or immunity.
The fact is that the mRNA is a very unstable molecule, has a very short half-life and is degraded immediately by scissor-like enzymes in the cytoplasm, called Ribonucleases or RNAses. Therefore, once the information from mRNA is translated into the protein, spike protein in this case, the viral mRNA will be degraded in no time. This means the material delivered through the COVID-19 vaccine does not stay in our system for long. High degree of instability associated with mRNA is one of the major reasons why the vaccine needs to be stored at ultra-low or sub-zero temperatures.
Secondly, the mRNA that is delivered into the cytoplasm via vaccination, cannot enter the nucleus where the DNA is residing. For the mRNA to enter the nucleus, it must be first converted to DNA (a process called “reverse transcription”) and then integrate into our DNA. However, the enzyme responsible for the conversion of mRNA to DNA, called “Reverse Transcriptase” and the enzyme responsible for integration of this converted DNA into our genome, “Integrase”, both are ABSENT in humans. Therefore, the mRNA delivered by the Pfizer/BioNTech vaccine into the cytoplasm remains spatially separated from our DNA (which is in the nucleus) and in no way the mRNA-based COVID-19 vaccine can alter our DNA.
In essence: COVID-19 vaccine does not affect or interact with our DNA in any way because:
- mRNA never enters the nucleus of the cell, which is where our DNA (genetic material) is kept.
- The cell breaks down and gets rid of the mRNA soon after it is finished translating the instruction.
Concern 3: The Pfizer-BioNTech COVID-19 vaccine is not effective.
Vaccines are more effective than almost any other medicine we use daily. Most people will be protected by most of the vaccines they receive, however, there will always be a small number of people who fail to make an immune response to a particular vaccine. If their body has not made an immune response, then those people remain vulnerable to the disease.
Most vaccines offer good protection for many years. For some vaccines it is necessary to give repeated doses of vaccines or booster doses to provide continued protection. But vaccines do not usually provide protection ‘forever’. Levels of protection may naturally decrease over time, or may be reduced because of medical conditions, medications, or aging, when the immune system may work less well.
In an FDA briefing document released on December 10th, 2020 for Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting, more details about the Pfizer COVID-19 vaccine were released. Safety and efficacy data reveal that a total of 43,448 participants received injections: 21,720 with vaccine and 21,728 with placebo (control/mock/blank injection). There were 8 cases of COVID-19 in the vaccine group as opposed to 162 cases among those assigned to placebo; indicating that vaccine was 95% effective in preventing COVID-19 in people above 16 years or older (X-axis on the graph below depict the days after dose 1 and Y-axis is the COVID-19 incidence in placebo, blue line and vaccinated individuals, red line).
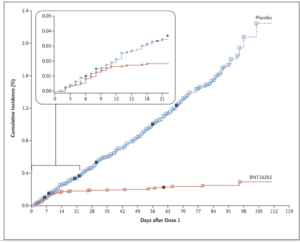 For comparison, the amount of virus neutralizing antibodies produced in the vaccinated individuals was found to be much higher than the antibodies produced in a person who had acquired COVID-19 infection naturally. This further indicates the efficacy of vaccination in providing immunity from natural infections in future. The trial included healthy people or even those who had stable chronic medical conditions, including but not limited to human immunodeficiency virus (HIV), hepatitis B virus, or hepatitis C virus infection. However, studies for future are planned to evaluate the efficacy of vaccine in pregnant women, children younger than 12 years, and those in special risk groups, such as immunocompromised individuals.
For comparison, the amount of virus neutralizing antibodies produced in the vaccinated individuals was found to be much higher than the antibodies produced in a person who had acquired COVID-19 infection naturally. This further indicates the efficacy of vaccination in providing immunity from natural infections in future. The trial included healthy people or even those who had stable chronic medical conditions, including but not limited to human immunodeficiency virus (HIV), hepatitis B virus, or hepatitis C virus infection. However, studies for future are planned to evaluate the efficacy of vaccine in pregnant women, children younger than 12 years, and those in special risk groups, such as immunocompromised individuals.
The data also did not raise any specific safety concerns. The most common solicited adverse reactions were injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%); severe adverse reactions occurred in 0.0% to 4.6% of participants, were more frequent after Dose 2 than after Dose 1 and were generally less frequent in participants ≥55 years of age (≤ 2.8%) as compared to younger participants (≤4.6%).
Therefore, based on the totality of scientific evidence available, including data from adequate and well- controlled trials, it is reasonable to believe that the Pfizer-BioNTech COVID-19 vaccine will be effective in preventing COVID-19 in individuals 16 years of age and older, and the known and potential benefits of the Pfizer-BioNTech COVID-19 vaccine outweigh its the potential risks.
Concern 4: COVID-19 vaccine had caused severe life-threatening complications including face paralysis and death during the trials, but the vaccine was still approved and released in the market.
Bell’s palsy (facial paralysis caused by impairment of the seventh cranial nerve, one of the facial nerves) was reported by four vaccine participants and none in the placebo group. However, no correlation or expected causal relationship has been established between the vaccine and Bell’s palsy. The normal incidence of Bell’s palsy is roughly 20 people out of 100,000. The Pfizer study examined roughly 40,000 people, so four cases are within the normal observed incidence of Bell’s palsy and could be simply coincidental. Dr. Sara Oliver, an Epidemic Intelligence Service officer with the Centers for Disease Control and Prevention’s Division of Viral Diseases, said there is “no known or expected causal relationship between the vaccine and Bell’s palsy.” A similar issue arose decades ago, when a few isolated cases of people developed Bell’s palsy after getting a flu vaccine. However, no study has ever established a link between the flu vaccine and Bell’s palsy.
A total of six deaths occurred during the trial period [2 deaths in the vaccine group (n=2), 4 in the placebo group (n=4)]. In the vaccine group, one participant with baseline obesity and pre-existing atherosclerosis died 3 days after Dose 1, and the other participant experienced cardiac arrest 60 days after Dose 2 and died 3 days later. Of the four deaths in the placebo arm, the cause was unknown for the two of them, and the other two participants died of hemorrhagic stroke (n=1) and myocardial infarction (n=1), respectively.
Concern 5: COVID-19 vaccine will give me COVID-19.
No. The Pfizer-BioNTech COVID-19 vaccine does not contain SARS-CoV-2/COVID-19 virus and cannot give COVID-19. The vaccine only contains a small piece of genetic information in the form of mRNA that can code for the viral spike protein. This protein by itself is innocuous and since the vaccine does not contain any live virus or even its parts, it is completely safe from that perspective.
Concern 6: FDA approved Pfizer-BioNTech COVID-19 vaccine has HIV.
The Pfizer vaccine has no component that can give HIV to the recipients. The vaccine is purely based on mRNA delivery that contains information or instructions for the cells to produce only the spike protein of the COVID-19 virus.
Recently there are videos circulated on social media about COVID-19 vaccine giving HIV to the recipients. As a matter of fact, this trouble arose with the in-house produced Australian vaccine, developed by the University of Queensland (UQ) and the biotech company CSL Limited, a Melbourne, Australia–based pharmaceutical firm. This Australian vaccine (based on “Clamp technology”) used a small fragment of HIV protein (80-amino acids) along with the spike protein of COVID-19. The researchers believed that this HIV protein will act as a clamp to stabilize the COVID-19 spike protein and allow the immune system to respond more effectively to the vaccine. Although the use of the HIV protein was not expected to pose any risk of HIV infection, the volunteers developed antibodies against the clamp portion of the vaccine in addition to the spike protein. This means that the volunteers had antibodies against HIV protein in their blood after they received the Australian vaccine. These volunteers thus did not contract HIV disease from the vaccine, all what they had was the circulating anti-HIV antibodies as a result of vaccination. Since the HIV testing kit is based on the principle that if a person is infected with HIV, their blood will have circulating HIV antibodies, which will be detected by the kit. Therefore, it was not surprising that few COVID-19 vaccine volunteers tested falsely positive for HIV after receiving the Australian vaccine. However, even if they tested positive for HIV antibodies, this in no way implies that they acquired HIV disease from the COVID-19 vaccine. Having HIV antibodies in the system does not necessarily always mean active HIV disease.
Nevertheless, the locally produced Australian vaccine is no longer in use and was abandoned right after Phase I trial, which was performed in 216 volunteers, for the reasons explained above. Further, the Australian vaccine has no similarity with the FDA approved Pfizer-BioNTech vaccine. Both these vaccines have entirely different mechanisms of action. We cannot compare apples with the oranges.
Concern 7: The COVID-19 vaccine cannot give long-term protection because the COVID-19 virus is rapidly mutating/changing. New strain has emerged in the UK, which is more contagious, and the present day approved vaccine will not be able to protect from the infection with this new strain.
For vaccines to work, the strain of bacteria or virus in the vaccine needs to be the same as the strain that causes disease in the population (or at least very similar to it). Some viruses and bacteria change over time, and this can have an impact on how effective vaccines are. For example, the flu virus can change very rapidly, meaning that last year’s flu vaccine is unlikely to protect us from the virus strains that are circulating this year. Therefore, every year pharmaceutical companies have to come up with a revised flu shot that well responds to the mutated virus and we are again required to take a flu shot. In contrast, the measles virus hardly changes from year to year, and so the measles vaccine that forms part of the MMR is as likely to protect us today as it was ten years ago.
Although COVID-19 was only discovered in humans recently, mutations in the gene encoding Spike (S) protein (target protein used in the COVID-19 vaccine) are being continuously reported. Out of several mutations in the Spike protein, the frequency of one that replaces aspartic acid at the 614th position (D614) to glycine (G614) was increased rapidly in April and May. The increasing predominance of the G614 virus in the human population raises the possibility that viruses with this mutation have a fitness advantage, perhaps allowing more efficient person-to-person transmission.
From the publicly available genome information on the viral sequences that were circulating in the population through the end of March 2020, the Pfizer-BioNTech has tested its vaccine for protection against 19 such mutated viral strains including the dominant G614 strain. The vaccine was found to generate neutralizing antibody response against all the circulating viral strains similar to the wild-type/unmutated COVID-19 virus, demonstrating the breadth of the protection it can afford against even the newly emerged strains.
Regarding the newly emerged strain in the UK, there is no scientific research data available yet that confirms the virus to be more contagious or transmissible. Also “Given the small fraction of US infections that have been sequenced (51,000 of the 17 million cases), the variant could already be in the United States without having been detected,” said the CDC. It is also premature to come to any conclusions regarding the efficacy of the FDA approved vaccine for this new strain.
Concern 8: COVID-19 vaccine is not safe, it can cause injury, reversals, and adverse reactions.
Polls show that as many as 42 percent of Americans say they are unwilling to get a COVID-19 vaccine when one becomes available, for reasons spanning the unprecedented speed of the vaccines’ development to a general mistrust of vaccines. People are also concerned about potential side effects from the shots. Like any other pharmaceutical product, vaccines are also not completely risk-free. WHO states, “There is no such thing as a “perfect” vaccine which protects everyone who receives it and is entirely safe for everyone”. Therefore, adverse events might occur post-vaccination. A major reason why the public has an overall low or zero tolerance towards even a minor adverse event following vaccination is because vaccines are given to healthy people to prevent disease, unlike medications (antibiotics, insulin etc) that are administered to the sick people. For this reason, a higher standard of safety is expected for vaccines in comparison to the other pharmaceutical products.
An Adverse Event Following Immunization (AEFI) is defined as any untoward medical occurrence that follows immunization. AEFIs can be minor (like fever, local pain, redness and swelling at the injection site), moderate (like pain and swelling which spreads beyond the nearest joint or high-grade fever) or major/severe (conditions requiring hospitalization or leading to death or disability).
AEFIs are divided into 5 categories:
(a) Vaccine product-related reaction
An AEFI caused by one or more of the inherent components present in the vaccine itself. Example: extensive limb swelling or high-grade fever following a DTP shot (1 in 1,000,000), a rash/purple bruise after MMR (1 in 20,000).
However, COVID-19 vaccine does not contain any such ingredient that is known to be toxic. It only contains mRNA, lipids, salts and sugar.
(b) Vaccine quality defect-related reaction
An AEFI that is caused because of the manufacturing defect leading to the production of a poor-quality vaccine. Example: Incomplete inactivation of polio virus in the vaccine can lead to paralytic polio.
However, COVID-19 vaccine does not contain live or even dead virus, that can potentially revert and cause disease.
(c) Immunization error-related reaction
An AEFI that is caused by human error and thus by nature, this type of AEFI is preventable. Most common human errors are inappropriate vaccine handling, prescribing or administration. Example: transmission of infection by contaminated multi-dose vials (vaccine vial that contains more than one dose is susceptible to contamination due to malpractices in handling the vial), improper sterilization or re-use of syringes/needles, injection at a wrong site, improper vaccine storage or transport or ignoring the contraindications (e.g., person’s past history of allergies).
COVID-19 vaccine is supplied as a multi-dose vial, with each vial containing dose for 5 people. Therefore, like any other medical procedure, it is essential to exercise caution while using and storing the vials.
(d) Immunization anxiety-related reaction
An AEFI arising from anxiety about immunization. Example: Vasovagal syncope (nervous reaction leading to a fainting spell) during/following vaccination.
(e) Coincidental or unknown events
An AEFI that is caused by something other than the four causes (vaccine components, its quality, immunization error or immunization anxiety) mentioned above. Example: a fever occurs at the time of the vaccination but in fact is caused by malaria. Coincidental events mean occurrence of two or more events around the same time, but the preceding event may or may not be causally related to the later one.
Effective vaccines may produce some undesirable side effects which are mostly mild and clear up quickly. Most events thought to be related to the administration of a vaccine are actually not due to the vaccine itself – many are simply coincidental events and others are due to human, or program, error (particularly in developing countries).
As of December 18th, there have been only six reports of allergic reactions to the Pfizer-BioNTech shot in the U.S., according to the CDC. More than 272,000 doses of the vaccine had been administered by that time. It is not possible to predict how everyone would respond to a vaccine and who would develop a mild or a serious reaction post-immunization. But there are a few contraindications to some vaccines. By following those contraindications, the risk of serious adverse effects can be either minimized or mitigated completely. CDC recommends that people with a history of severe allergic reactions not related to vaccines or injectable medications—such as allergies to food, pet, venom, environmental, or latex—may still get vaccinated. People with a history of allergies to oral medications or a family history of severe allergic reactions, or who might have a milder allergy to vaccines (no anaphylaxis)—may also still get vaccinated. CDC states that if you have had a severe allergic reaction to other vaccines or injectable therapies, you should ask your doctor if you should get a COVID-19 vaccine. Your doctor will help you decide if it is safe for you to get vaccinated.
Bibliography
- Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine; F.P. Polack, et al. DOI: 10.1056/NEJMoa2034577
- Zhang L et al, SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity, Nature Communicationsvolume 11, Article number: 6013 (2020)
- Sahin U et al, BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. doi:https://doi.org/10.1101/2020.12.09.20245175
- Dawood, A.A. (2020). Mutated COVID-19, May Foretells Mankind in a Great Risk in the Future. New Microbes New Infect. 35, 100673.
- Saha, P., Banerjee, A.K., Tripathi, P.P., Srivastava, A.K., and Ray, U. (2020). A virus that has gone viral: amino acid mutation in S protein of Indian isolate of Coronavirus COVID-19 might impact receptor binding, and thus, infectivity. Biosci. Rep. 40, BSR20201312.
- Korber, B., Fischer, W.M., Gnanakaran, S., Yoon, H., Theiler, J., Abfalterer, W., Hengartner, N., Giorgi, E.E., Bhattacharya, T., Foley, B., et al. (2020). Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. Published online July 3, 2020. https://doi.org/10. 1016/j.cell.2020.06.043.
- van Dorp, L., Acman, M., Richard, D., Shaw, L.P., Ford, C.E., Ormond, L., Owen, C.J., Pang, J., Tan, C.C.S., Boshier, F.A.T., et al. (2020). Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 83, 104351.
- Sheikh, J.A., Singh, J., Singh, H., Jamal, S., Khubaib, M., Kohli, S., Dobrindt, U., Rahman, S.A., Ehtesham, N.Z., and Hasnain, S.E. (2020). Emerging genetic diversity among clinical isolates of SARS-CoV-2: Lessons for today. Infect. Genet. Evol. 84, 104330.
- Baum, A. et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science369, 1014–1018 (2020).